Computerized System Validation
We drive you to GxP compliance
Computer-aided systems that have an impact on product quality, patient safety and data integrity must be validated within the GxP environment (medical technology, pharmaceutical & life science sectors). The validated state must be maintained for the entirety of the system lifecycle. Ensuring a validated state can be extremely challenging for a company as the specific expertise may not be available to approach it pragmatically and efficiently. We can help you consistently and reproducibly ensure that your computer-aided systems are GxP compliant.
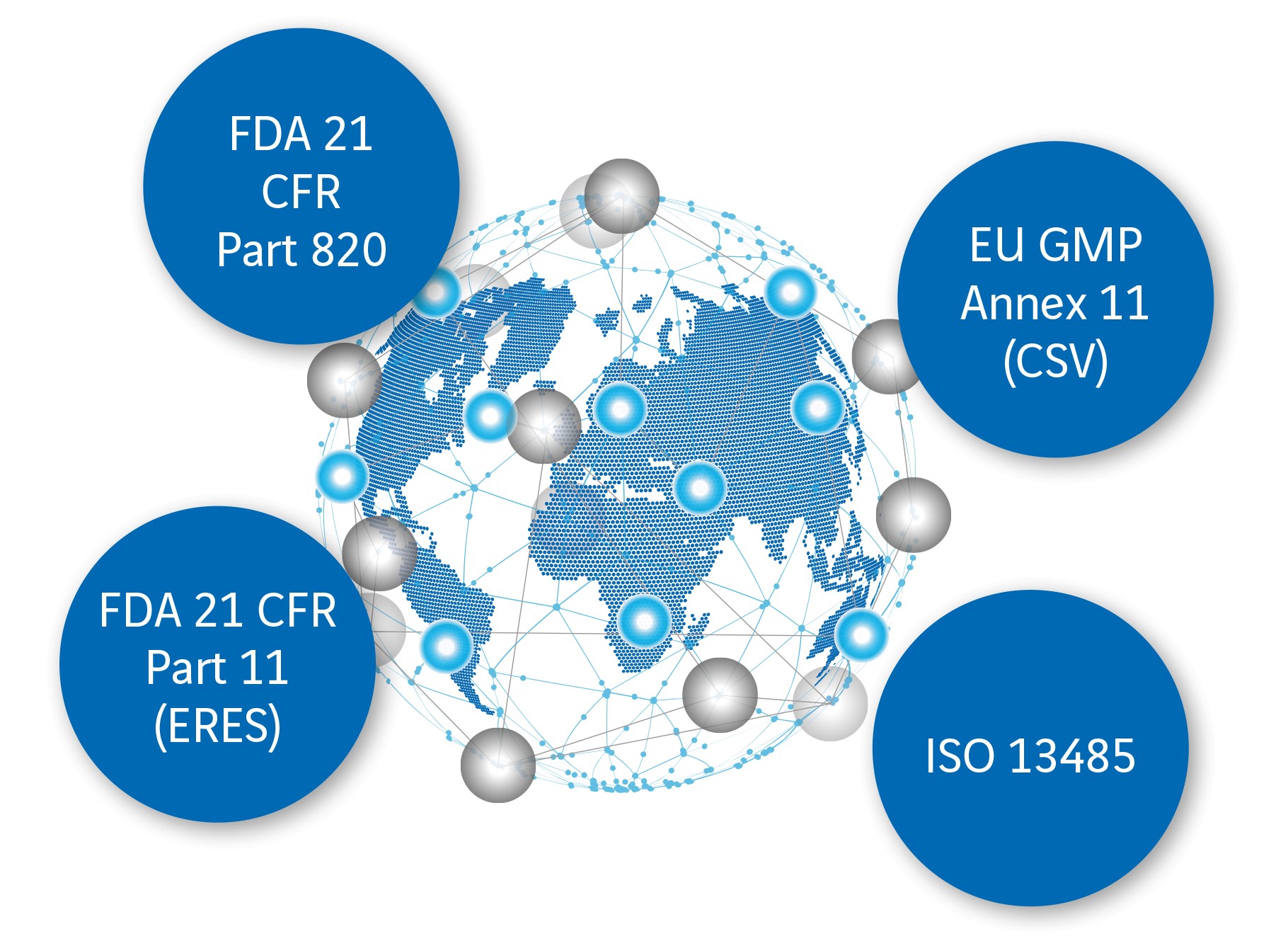
Compliance with guidelines and laws during validation
One of the challenges in validating computer-aided systems is compliance with, and expertise in, the following regulations and legislation:
- EU Directives and the quality management standard ISO 13485
- national pharmaceutical legislation
- American FDA (Food and Drug Administration) requirements
- Regulations from 21 CFR Part 820, 21 CFR Part 11 and EU GMP Annex 11
Validation of systems usually takes place in accordance with GAMP®5 industrial standards.
We provide appropriate solutions
Consulting, Validation as a Service or Greenfield
Streamlined, smart processes and a risk-based approach are the key to cost-effective computerized system validation. With our end-to-end solution, we can support our customers to consistently and reproducibly ensure that computer-aided systems are GxP compliant. In this case, our service is available as an ongoing service, on a project basis, or as a consulting contract.
-
Consulting
-
Validation as a Service
-
Greenfield

Computerized System Validation Factsheet
We can help you consistently and reproducibly ensure that your computer-aided systems are GxP compliant.
Contact us now and benefit from tailor-made solutions!
Our service is available as an ongoing service, on a project basis, or as a consulting contract.